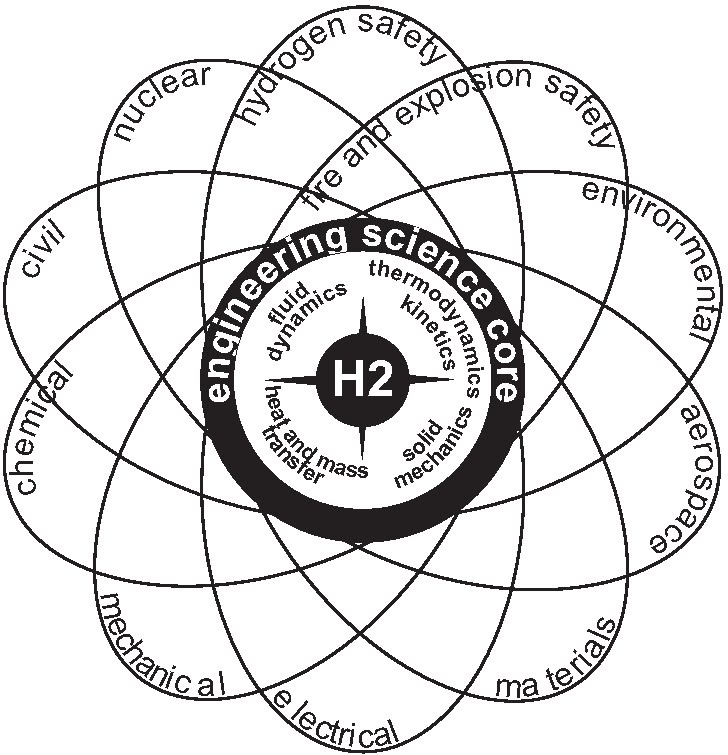
 |
| Figure 1: Hydrogen Safety in relation to other Branches of Engineering Science. |
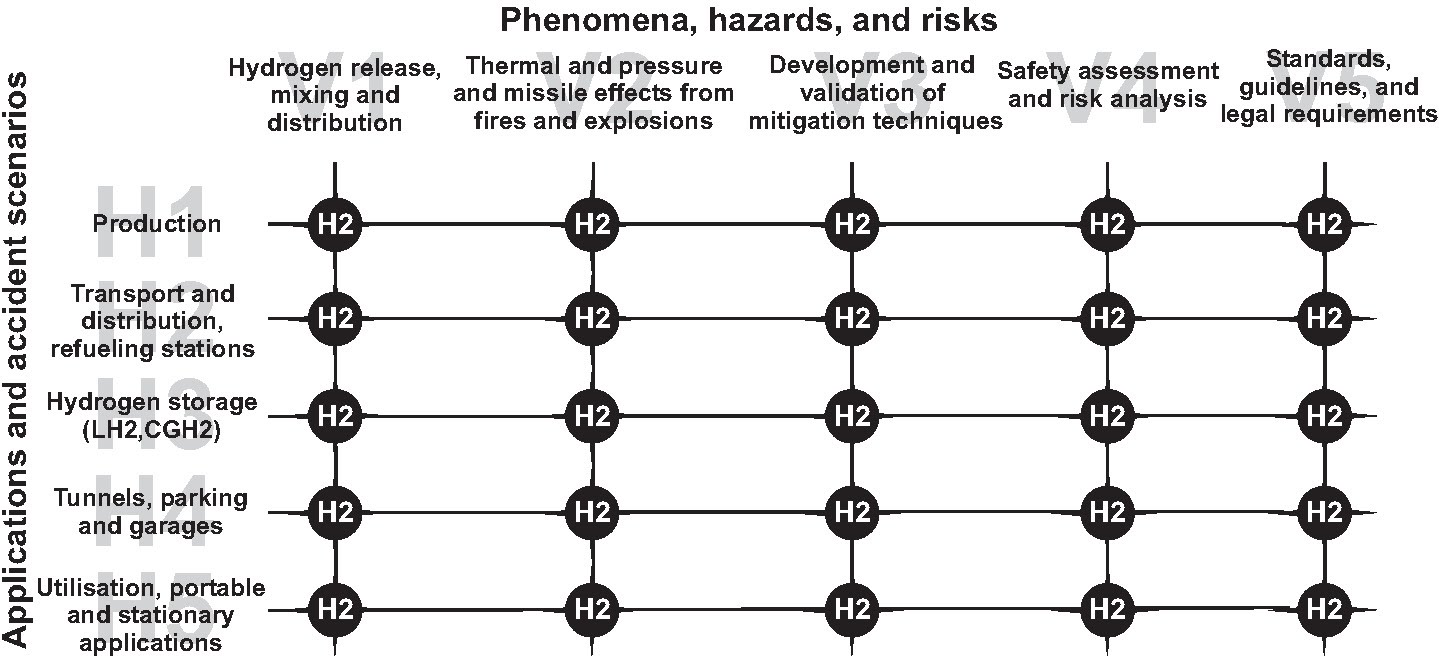 |
| Figure 2: The HySafe Activity Matrix. |
- Provide the student with an awareness of current problems (accidental releases, effects of fires and explosions, etc.) in application areas of the hydrogen economy (production, transportation, storage, utilisation, etc.).
- Provide the student with an understanding of theories, methodologies and paradigms that form the principles of hydrogen safety so that they may undertake his/her own research or advanced scholarship.
- Develop in the student a capability for independent learning to expand his/her knowledge in the principles of hydrogen safety engineering, and to understand how the boundaries of knowledge in this field are advanced through research.
- Provide the student with a conceptual understanding of the principles of hydrogen safety engineering so that he/she will be able to critically evaluate and use research information on accidental hydrogen releases, fires and explosions, material compatibility, etc. for the provision of hydrogen safety.
- Develop in the student the quality of originality in the application the principles of hydrogen safety engineering, together with a practical understanding of how established techniques of research and enquiry are used to create and interpret knowledge in specialist areas of hydrogen safety.
- Develop in the student the ability to deal with complex hydrogen safety engineering issues involving accidental hydrogen releases and dispersion, fires and explosions, material compatibility, etc. by applying the principles of hydrogen safety.
- On-line lectures.
- Communication Tools (video broadcasts, on-line fora, email tools, chat rooms, virtual whiteboard).
- Self-assessment Tools (student self-evaluation & timed on-line quizzes).
- Research Tools (external references for directed reading & search facilities).
- Navigation Tools (page annotation, session resumption, searchable image archive, linked searchable glossary, indexing).
- Asynchronous modes of communication are utilized throughout the entire course delivery.
Thermodynamical, Physical, Chemical and Safety Properties of Hydrogen
Atomic structure of a hydrogen molecule and safety related consequences: spin of the atomic nucleus, ortho-hydrogen, para-hydrogen, equilibrium between ortho-hydrogen and para-hydrogen (temperature dependence of the equilibrium, normal hydrogen), the release of heat accompanying ortho- to para-conversion, rate of the non-catalytic ortho- to para-hydrogen conversion. Safety issues connected to the storage of unconverted liquefied hydrogen (evaporation due to conversion heat release, possible hazard due to naturally occurring deuterium). Comparison between safety-related physical and thermophysical properties of normal- and para-hydrogen. States of matter (gaseous hydrogen, liquid hydrogen, solid hydrogen and other states of matter). Gaseous (GH2), liquefied (LH2) and slush (SLH2) forms of hydrogen. Phase diagram of hydrogen (PT-diagram): comparison with the general phase diagram of a pure substance, phase boundaries (boiling point (safety issues connected to the low boiling point of hydrogen), melting point, vapour pressure, sublimation vapour pressure, fusion curve, sublimation curve, vaporisation curve (the Clausius-Clapeyron equation), triple point, critical point, critical properties), composition of the PT-diagram (solid region, liquid region, vapour region, gas region, fluid region). Temperature dependence of vapour pressure. The density of hydrogen (gaseous hydrogen (the compressibility factor, corresponding states principle, prediction of the density: ideal gas law, van der Waals equation of state, the Nobel-Abel equation of state, the Beattie-Bridgeman equation of state), liquid hydrogen (prediction of the density: Rackett's correlation, the Lydersen-Greenkorn-Hougen correlation), safety issues arising from the density of hydrogen), Sonic velocity. Diffusivity (safety problems arising from the diffusivity of hydrogen). Viscosity. Thermal conductivity. Joule-Thompson inversion temperature. Phase equilibrium: dew point, bubble point; Raoult's law; Henry's law; K-value correlations. Properties connected to fire and explosion hazards: phenomenology of fires (diffusion flame, premixed flame, flash fire, jet fire), deflagrations, detonations (deflagration to detonation transition); adiabatic flame temperature, minimum spark ignition energy, flammability limits, flammability range of hydrogen and air, laminar burning velocity, critical charge for detonation initiation, detonation wave structure, detonation limits, detonation cell size (effect of equivalence ratio, comparison between hydrogen and hydrocarbon fuels), relationship between detonation cell width versus critical initiation energy for the onset of detonation, critical tube diameter for the onset of detonation (effect of equivalence ratio, comparison between hydrogen and hydrocarbon fuels). Health hazard properties: gaseous hydrogen (asphyxiant), liquefied hydrogen (cryogenic burns, frostbite, hypothermia, lung damage from inhalation of cold vapour). An overview of reported accidents and incidents caused by hydrogen embrittlement. Internal and external hydrogen embrittlement. States of hydrogen in steels: hydrogen in metallic solution, hydrogen in combined state. Gaseous hydrogen embrittlement: steel deterioration due to hydrogen in metallic solution, mechanism due to transport by dislocations, effect of temperature. Hydrogen attack: steel deterioration due to hydrogen in combined state, mechanism of formation of micro-cavities in the steel, effect of diffusional transport, effect of temperature. Influence of hydrogen pressure on crack growth rate. Test methods to investigate hydrogen embrittlement and hydrogen attack. Factors affecting hydrogen embrittlement: hydrogen purity, hydrogen partial pressure, temperature, exposure time, surface condition, nature of the material (critical concentration of hydrogen in the material, microstructure, chemical composition, mechanical properties). Mitigation of hydrogen embrittlement by the addition of vanadium and rare earth elements to ferritic steel, or, Ni, C, and Mn to austenitic stainless steels. Hydrogen embrittlement of other materials: brass and copper alloys, aluminum and aluminum alloys, Cu-Be (used in springs and membranes), Ni and high Ni alloys, Ti and Ti alloys. Mitigation of hydrogen attack: chemical composition (addition of Cr, Mo, Ti, W), heat treatment (stress relief treatment), level of stress (elimination of residual stresses by heat treatment).
Chemical Thermodynamics, Chemical Kinetics and Hydrogen Thermochemistry
Combustion reaction of hydrogen in air: stoichiometric equation, global versus elementary reactions, relationship between reaction rate and chemical species concentration, the three-parameter Arrhenius form to describe the reaction-rate constant (activation energy, temperature exponent, pre-exponential factor), overall reaction rate expression, overall reaction order (effect of equivalence ratio and pressure on overall reaction order), overall activation energy (effect of equivalence ratio and pressure on overall activation energy). Heats of reaction (constant pressure combustion: equality of reactant and product enthalpies; constant volume combustion: equality of reactant and product internal energies). Adiabatic flame temperature: the frozen flame temperature (absence of product dissociation), adiabatic flame temperature with product dissociation (equilibrium constants, chemical affinity and chemical potential, equilibrium as the condition of zero chemical affinity, chemical affinity as the partial molar Gibbs function, criteria for equilibrium (Gibbs free energy for constant pressure processes, Helmholtz free energy for constant volume processes)). Calculation of the adiabatic flame temperature by the element potential method (constant pressure combustion: minimisation of the Gibbs free energy; constant volume combustion: minimisation of the Helmholtz free energy; examples of chemical equilibrium codes (CANTERA); limitations imposed by the inclusion of the ideal gas law in chemical equilibrium codes; equations of state for high pressure effects up to 700 MPa: virial equations of state, Becker-Kistiakowsky-Wilson equation of state). Reaction mechanisms: forward elementary reactions, backward elementary reactions, the chemical equilibrium constant as the ratio between the forward and backward elementary reaction rates, detailed schemes (the Dougherty & Rabitz mechanism, the Miller, Mitchell, Smooke & Kee mechanism, the Marinov, Westbrook & Pitz mechanism, the O'Conaire, Curran, Simmie, Pitz & Westbrook mechanism, the Saxena & Williams mechanism), reduced mechanisms (example: a four-step reduced mechanism for hydrogen-air mixtures by Lu, Ju & Law). Chain branching: the concept of a chain carrier. Removal of chain carriers by a three-body collision with a third body. The crossover temperature. Falloff. The fall-off reaction rate: the Lindemann fall-off rate constant, the Stewart fall-off rate constant, the Troe fall-off rate constant. Chaperon efficiencies. Software tools for analysing detailed chemical kinetic mechanisms (CANTERA). Validation of kinetic mechanisms from critically-reviewed experiments including stretch-free laminar burning velocities, flow reactor species profiles, ignition delay times in shock tubes, etc. Surface reactions. Surface adsorption processes: relation to catalysis, improvement of the miners' safety lamp due to Henry in 1824 by the addition of platinum powder to the reacting surface, Faraday's view on the role of adsorption to the surface in catalysis, physiosorption, van der Waals adsorption, chemisorption, Langmuir's concept of the unimolecular layer, Langmuir's adsorption isotherm, monolayer adsorption, multi-layer adsorption, adsorption with dissociation, competitive adsorption. Surface reaction processes: reaction mechanism, the Langmuir-Hinselwood mechanism, the Langmuir-Rideal-Eley mechanism, the precursor mechanism, Unimolecular surface reactions. Bimolecular surface reactions. Desorption. Kinetic model of hydrogen-oxygen reaction on the platinum surface. Kinetic rates of hydrogen-oxygen reaction on the platinum surface. Application in hydrogen safety: the three explosion limits in the flammability diagram, dependence explosion limits of hydrogen-oxygen systems on containment shape, nature of surface, added inert gases (inertisation by steam), spontaneous ignition of hydrogen leaks. ignition by hot surfaces, catalytic recombiners, initial conditions for self-sustained detonation, boundary conditions for self-sustained detonation, prediction of detonation limits of hydrogen-air and hydrogen-oxygen mixtures, prevention of hydrogen ignition (electrical circuits, static electricity, hot surface, open fire, shock waves, (hot) gas jet, explosives, exothermic reaction, pyrophoric substances, lightning). Overview of hydrogen ignition mechanisms and relevant prevention techniques: electrical circuits, static electricity, hot surface, open fire, shock waves, (hot) gas jet, explosives, exothermic reaction, pyrophoric substances, lightning, etc. Autoignition and safety in hydrogen powered vehicles. Standard IEC 60079-10 'Electrical apparatus for explosive gas atmospheres - Part 10: Classification of hazardous areas'.
Fluid Dynamics, Reacting Flows and the Application of Simulation in Hydrogen Safety
The role of modeling and simulations for the provision of hydrogen safety. Overview of governing equations (instantaneous equations, Reynolds and Favre decomposition, filtering) and turbulence concepts (closure problem, Reynolds stresses, Boussinesq hypothesis, subgrid-scale stresses) and modeling (Prandtl mixing length model, the k-epsilon model, Reynolds stress models, LES models). The equations of change for turbulent reacting flows and closure models. Large Eddy Simulation: mass weighted Favre averaging and the filtered balance equations for (non)-reacting flows, sub-grid scale models. Brief overview of applications to practical hydrogen safety provision (garages, parking places, tunnels, re-fuelling stations, liquefied hydrogen storage, fuel-cell storage, bursts of high-pressure vehicle tanks, pressure-release devices, post-release mitigation, accidental combustion, stand-off distances).
Hydrogen Releases, Mixing and Dispersion
Molecular and turbulent mixing. Jet releases. Sonic and supersonic jet releases. Joule-Thompson inversion. Governing equations for jets. Laminar jets, plane and round jets, impinging jets. Turbulent jets: transition to turbulence, morphology of jet establishment. Scaling parameters for under-expanded supersonic jets. Buoyant jet in stably stratified surroundings: formation of a buoyant ceiling layer in an enclosure; steady state plume, puff and starting plume; plume formation distance and concentration profiles. Ventilation effects on the buoyant plume in an enclosure. Examples of CFD calculations for hydrogen dispersion in simple and complex enclosures: hydrogen releases in rectangular enclosures representative of residential garages; estimation of the hydrogen concentration during accidents in nuclear power plants (hydrogen generation and release during the Three Mile Island accident; simulation of the three dimensional behavior of a hydrogen-steam mixture within a subdivided containment volume following hydrogen generation during a severe accident in nuclear power plants). Boil off phenomenon. Cryogenic hydrogen spills: cryogenic spills and pool spreading; boiling modes, pool boiling, crisis of boiling, the Leidenfrost phenomenon, forced convection boiling, sub-cooled boiling, saturated boiling. Effect of boundary layer in atmosphere on dynamics of hydrogen cloud formation. Overview of experimental data and modelling of gaseous and liquefied hydrogen releases. Peculiarities of handling different types of releases: permeability, leaks and subsonic releases, high-momentum releases, cryogenic hydrogen spills, 'explosive' evaporation, catastrophic failures, boil off. Hydrogen detection and hydrogen sensors. Hydrogen removal: ventilation, thermal recombiners, passive autocatalytic recombiners. Preventive ignition of unscheduled releases: glow plug igniters, spark igniters, catalytic igniters.
Premixed Combustion of Hydrogen Mixtures
Laminar premixed flames: phenomenology, structure of the reaction zone, laminar burning velocity and laminar flame thickness. Stabilisation of laminar premixed flames on burners. Flash-back, blow-off and flame quenching. Effect of equivalence ratio, diluent concentration, pressure and temperature on the laminar burning velocity. Cellular flame structure and flame wrinkling. Effect of flame stretch and flame curvature on the laminar burning velocity. Turbulence generated by flame front itself. Turbulent premixed flames: phenomenology, turbulent flame brush, turbulent burning velocity and turbulent flame thickness. Turbulence scales and the interaction between turbulence and flames. The Borghi-diagram and interpretation of combustion regimes. The closure problem in turbulent premixed combustion. Flamelet models and flame surface density models. Flame extinction by turbulence.
Non-premixed and Partially Premixed Combustion of Hydrogen
Laminar diffusion flames: passive scalars, mixture fraction, flame structure in the mixture fraction space, state relationships, the Burke-Schumann flame structure, Laminar jet flames in a uniform flow field and flame length. Turbulent diffusion flames: relationship between flame height and fuel flow rate, stable lifted flames and blow-out phenomenon, dependence of flame length and shape on jet direction, correlation between flame length and rate of heat release. Partially premixed combustion: triple flames, combustion of an inhomogeneous mixture in a closed vessel and pressure build up. Prediction of jet fire parameters: temperature, visibility, flame length and flame shape, radiation. Pool fire characteristics. Fireball characteristics. Case studies and analysis of experimental data on thermal effects of hydrogen fires. Thermal effects on people and construction elements: tolerance limits, fire resistance rating. Damage criteria for buildings,
vehicles and people. Safety distances for hydrogen fires.
Deflagrations and their mitigation
Phenomenology of deflagration. Explosion severity parameters: relationship between explosion severity parameters and flame propagation parameters, pressure and temperature dependence of explosion severity parameters, effect of obstacles on flame propagation, flame acceleration and pressure build up. Confined deflagrations: dynamics of flame front propagation, flame induced flow, flame instabilities and flame wrinkling, prediction of pressure build-up in closed space, the Mache effect. Unconfined large-scale deflagration dynamics: mechanisms of flame propagation acceleration and the role of instabilities, positive and negative phases of pressure dynamics, pressure wave decay in the atmosphere. Overview of hydrogen deflagration mitigation techniques : pressure containment, deflagration venting, suppressant barriers, suppressant injections, fast-acting valves, flame front diverters, inherently safe design, inertisation, deflagration flame arresters, quenching diameter, dependence of the quenching diameter on pressure and application in deflagration flame arresters, quenching on the wall.
Detonations
Phenomenology of detonation. The Hugoniot curve: the Hugoniot relations, the Rankine-Hugoniot relation, the Rankine-Hugoniot diagram, the Rayleigh-line relation, the Chapman-Jouget points, the Chapman-Jouget detonation wave velocity. The detonation wave structure: the Zeldovich-von Neumann-Doring theory of detonation (one-dimensional wave structure), three-dimensional detonation wave structure. Detonation limits: confined and unconfined detonation limits, comparison between different fuels, effect of a problem scale. Detonation cell size: dependence on composition, temperature and pressure, comparison between hydrogen and hydrocarbon fuels, relationship between detonation initiation energy and detonation cell size, comparison between hydrogen, other fuels, and explosives, critical tube diameter for the onset of detonation. Deflagration to detonation transition (DDT): phenomenology of flame acceleration and DDT; effect of chemical composition, pressure, temperature, geometry, and physical size of the system. Autoignition delay times for hydrogen-air mixtures. Possible measures for reducing the potential of detonation wave generation: inhibition of flames, venting in the early stages of an explosion, quenching of the flame-shock complex, detonation flame arresters.
Pressure Effects of Hydrogen Explosions, Structural Response, Fragmentation and Missile Effects
Structural response to explosion loadings: amplification factors for sinusoidal and blast loadings, P-I diagrams for ideal blast sources and non-ideal explosions, energy solutions, dimensionless P-I diagrams. Structural response times for plates. Damage criteria for buildings, vehicles and people. Fragmentation and missile effects: primary and secondary fragments; drag-type and lifting-type fragments; impact effects; trajectories and impact conditions.
Risk Assessment Methodologies, the Regulatory Framework, and Safety Standards related to Hydrogen Applications
Terms and definitions: hazard, danger, accident, risk, risk analysis, risk assessment, etc. Origins and a brief history of risk analysis and loss prevention. Basic factors determining hazard and risk of substances. Hydrogen safety and regulatory issues: safety issues, public acceptance and safety, regulatory issues. Approval process: the example of hydrogen road vehicles, the case of hydrogen refuelling stations. Introduction to modern safety philosophy: the modern risk-based approach (see Figure 3) to the management and regulation of safety; an introduction to the important components of risk, i.e. hazards, likelihood, consequence and hazardous event; how an understanding of these provides a basis for reducing risk and increasing safety. A brief overview of a modern structured approach to managing the risk from hydrogen. The chain: potential, trigger of cause-consequences, exposed vulnerable elements and the design actions for safety both technical and organizational in inherent safety, prevention, containment etc. An introduction to risk assessment and the goal-setting basis of modern legislation. Hazard identification and analysis methods. Hazard ranking methods, the Dow Fire and Explosion Index. Hazard and operability studies (HAZOP). Consequence analysis. Dispersion and transmission models: the structure of the atmosphere and its relation to transmission and plume behaviour. Dispersion models: critical Richardson number criterion, the Gaussian plume model, dispersion from a free turbulent gas jet, etc. Vulnerability and damage: general response function given intensity of effect and time of exposure, fires and dose-response of heat radiation exposure, blast wave strength from vapour cloud explosion, blast interaction with objects, damage caused by blast waves, blast effects on people, toxic effects, domino effects. Failure frequency estimation. Fault tree analysis (FTA): minimum cut sets. Risk presentation, acceptance criteria and perception (individual and group risk and their application to "external or public safety"; uncertainty in risk assessment). Risk reduction and control: safety management system (SMS), history of accident frequency, the crucial role of management and human factor, accident investigation. Risk reducing measures: rapid ranking and the risk matrix, layer of protection analysis (LOPA), safety instrumented systems (SIS), other protective measures, maintenance. Design methods and design safety reviews. The costs of accidents. The costs of safety: investment and profitability, cost optimisation, loss of life, the law of large numbers, limited scope - selection of alternatives. Use of CFD in risk assessment. Hydrogen safety and regulatory issues. Public acceptance and safety. Safety legislation: hierarchy in safety legislation; purpose of safety legislation (imposing duties, responsibilities and accountabilities on people and organizations); the meaning of codes, standards, guidance and regulations; the origin of codes (developed by industry or trade bodies), standards (developed by engineering or standard bodies), and regulations (issued by the State); the Approved Code of Practice. A detailed examination of the structured approach to safety demanded by the ATEX Directives (substitution, preventing the formation of explosive atmospheres, containment, dilution through effective ventilation, preventing the ignition of explosive atmospheres, zone classification, mitigating the effects of an explosion, use of explosion resistant equipment, explosion relief, explosion suppression, prevention of explosion propagation, organizational measures to ensure explosion protection). Trans-national nature of safety regulations, codes and standards (RCS). Key European safety legislation that applies to hydrogen: EU ATEX Directives (ATEX 100 (Product Directive) and ATEX 137 (User Directive)). Compliance of the EU ATEX Directives with the EMC Directive 89/336/EEC (modified by 92/31/EEC and 93/68/EEC (the CE Marking Directive)), the Machine Directive 98/37/EC, Pressure Vessel Directive 97/23/EC, and, the Low Voltage Directive 73/23/EEC. IEC Standard 61511: structure (Part 1: Framework, definitions, system, hardware and software requirements; Part 2: Guidelines in the application of IEC 61511-1; Part 3: Guidance for the determination of the required safety integrity levels), and, harmonisation (adoption of IEC 61511 as EN 61511, by the European standards body CENELEC; IEC 61511 not harmonized under any Directive of the European Commission), purpose, and, scope, structure, scope (basic functional safety standard applicable to all kinds of industry), and, paradigm (risk is defined as function of frequency (or likelihood) of the hazardous event and the event consequence severity; zero risk can never be reached, safety must be considered from the beginning, and, non-tolerable risks must be reduced (ALARP)). Examples of how codes, standards and guidance may be used to manage risk and comply with the law. Approval of new hydrogen technologies by RCS (HyApproval WP2: Handbook for hydrogen refuelling station approval). Standards: ISO TR 15916(E) - Basic considerations for the safety of hydrogen systems; ISO DIS16110-1 - Hydrogen generators using fuel processing technologies; ISO DIS/CD 16111 - Transportable gas storage devices - Hydrogen absorbed in reversible metal hydride; ISO DIS22734-1 - Hydrogen generators using water electrolysis process; ISO FDIS17268:2006(E) - Compressed hydrogen surface vehicle refuelling connection devices; ISO PDTS 20012 - Gaseous hydrogen - Fuelling stations.
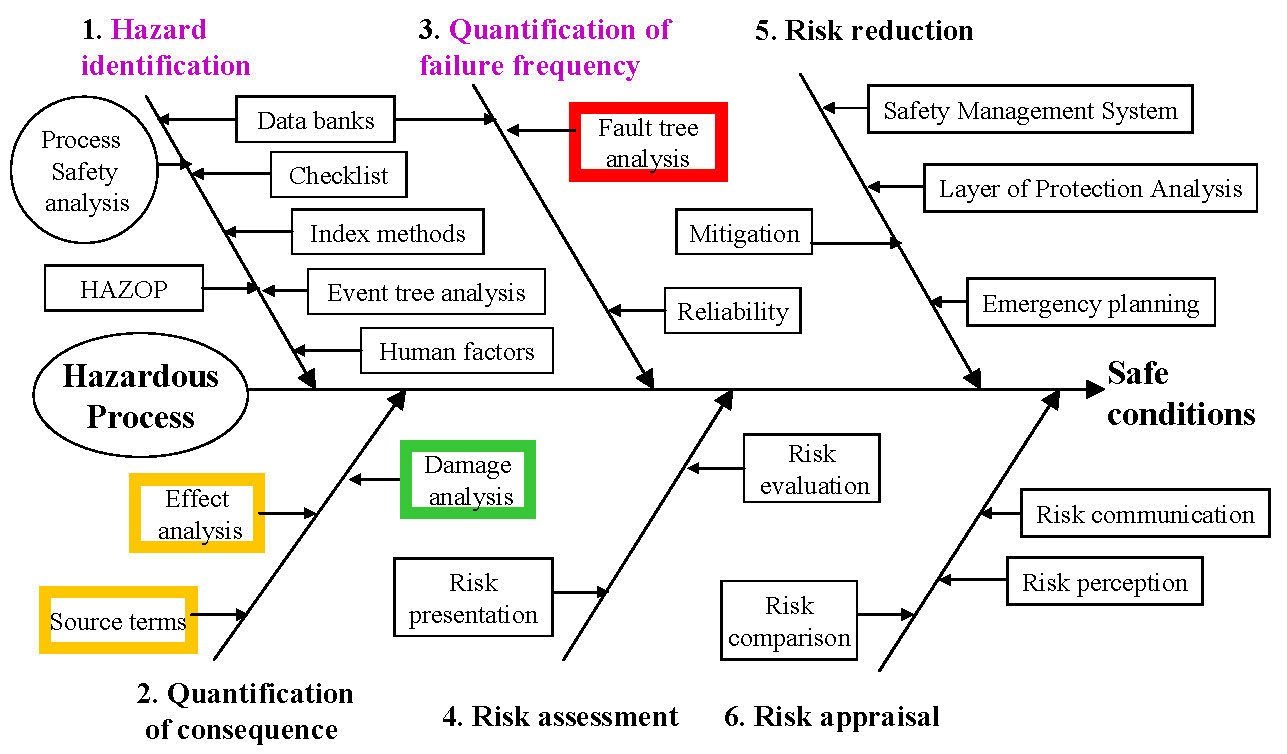 |
| Figure 3: The Risk Analysis Fishbone Diagram. |
It is attempted to implement the concept known as constructive alignment. Constructive alignment is a linguistic contraction between constructivism and instructional alignment of assessment elements. Constructivism is a phenomenon whereby the human mind generates new knowledge and problem solving skills from a pre-existing knowledge base and skill set. The method to achieve this is by a combination of instructional alignment of assessment elements and formative feedback. The lectures of the course are developed in such a manner that each lecture builds upon all previous lectures so that the learning curve increases progressively along the levels of cognitive complexity (see Table 1). Each lecture has its own assessment in the form of an on-line quiz that needs to be completed successfully at the end of each lecture prior to progressing onto the next lecture.
A progressively increasing learning curve is ensured by two coursework assignments, each consisting of five questions (with sub-questions).
Students solve the coursework questions as they progress through the course. These questions are designed in such a manner that students need to master the content of all previous lectures as they progress with the coursework.
Student learning and the achievement in the learning outcomes is monitored via the Discussion Forum. The Discussion Forum is organized in such a manner that each sub-question of the coursework can be discussed separately by staff-student feedback and student-student interaction.
The coursework assignments are designed to consist of the following elements:
(i) Explaining subject related concepts (25%)
(ii) Solving closed-ended problems (25%)
(iii) Solving open-ended problems (25%)
(iv) Creating solutions to complex problems (25%)
The occurrence of constructivism can then be observed and quantified from aforementioned elements (iii) and (iv) of the coursework submittals.
Two coursework assignments assess the learning outcomes listed below. Each coursework assignment consists of five questions (20 marks each), each comprising sub-questions. Questions may include short essays, tests of factual knowledge, and problem solving. Where possible, problems encountered in the working environment of students are integrated into the coursework assignments. Each coursework assignment contributes 50% to the overall grade.
- The first coursework assignment measures the student's achievements in learning outcomes K1, K2, K3, I1, I2, I3, P1, P5, T1, T2, and T3.
- The second coursework assignment measures the student's achievements in learning outcomes K4, K5, I4, P2, P3, P4, P5, T1, T2, and T3.
Successful students will be able to:
KNOWLEDGE AND UNDERSTANDING
- K1 Explain the vulnerability of the transition towards a hydrogen economy to safety issues
- K2 Describe the thermodynamics and kinetics of the hydrogen-air combustion reaction
- K3 Describe the various releases of compressed gaseous and liquefied hydrogen
- K4 Explain the different modes of turbulent hydrogen combustion: premixed, non-premixed, and partially premixed
- K5 Point out the features of the multi-dimensional detonation wave structure and identify detonation cell size as a design parameter for safety
INTELLECTUAL QUALITIES
- I1 Assess appropriate equations of state to explain the non-ideal pressure-temperature-density behaviour of gaseous hydrogen
- I2 Analyse the hydrogen combustion reaction in air and its application to deflagration and detonation waves
- I3 Appraise the phenomenology of hydrogen jet releases and liquefied hydrogen spills
- I4 Appraise the application of turbulent combustion modelling to hydrogen explosions
PROFESSIONAL/PRACTICAL SKILLS
- P1 Estimate the hydrogen concentration after accidental releases and set back distances
- P2 Predict jet fire parameters for hydrogen fires while taking thermal effects on people and construction elements, and, damage criteria for buildings, vehicles and people into account
- P3 Calculate pressure effects of hydrogen explosions, determine safety distances to protect people and structures against pressure effects, and, design mitigation techniques
- P4 Demonstrate expertise in assessing possible measures for reducing the potential of detonation wave generation (inhibition of flames, venting in the early stages of an explosion, quenching of the flame-shock complex, detonation flame arresters)
- P5 Evaluate CFD calculations of hydrogen safety problems involving jet releases of compressed hydrogen gas, liquid hydrogen spills, and confined/unconfined hydrogen deflagrations
TRANSFERABLE SKILLS
- T1 Display mastery in analysing complex hydrogen safety problems both systematically and creatively, by integrating fundamental knowledge and engineering approaches from a variety of disciplines, and communicate their conclusions to specialist and non-specialist audiences
- T2 Demonstrate self-direction and originality in tackling and solving hydrogen safety problems at a professional or equivalent level
- T3 Undertake advanced scholarship in the principles of hydrogen safety
| KEYWORDS FOR THE LEVEL OF COGNITIVE COMPLEXITY OF LEARNING OUTCOMES | |||||
| Knowledge & Understanding | Intellectual Skills | ||||
Knowledge Recalling important information |
Comprehension Explaining important information |
Application Solving closed-ended problems |
Analysis Solving open-ended problems |
Synthesis Creating solutions to problems |
Evaluation Making critical judgments based on a sound knowledge base |
| define list name recall record relate repeat underline |
derive describe discuss explain express identify locate recognize report restate review tell translate |
apply demonstrate dramatize employ illustrate interpret operate practize schedule sketch use |
analyse appraise calculate categorize compare contrast criticize debate diagram differentiate distinguish examine experiment inspect question relate solve test |
arrange assemble collect compose construct create design formulate integrate manage organize plan prepare propose set up |
appraise assess estimate compare evaluate judge rate revise |
Lecture notes based on the book manuscript Dahoe AE: Principles of Hydrogen Safety.
The references listed in this section are pointers to literature cited by the required reading.
Aceves, S.M., Berry, G.D. & Rambach, G.D. (1998) Insulated pressure vessels for hydrogen storage on vehicles. International Journal of Hydrogen Energy, 23, pp.583-591.
Ahmad, Z. (2006) Principles of corrosion engineering and corrosion control. Butterworth-Heinemann/IChemE Series. Amsterdam, Elsevier.
AIChE CCPS. (1994) Guidelines for evaluating the characteristics of vapor cloud explosions, flash fires, and bleves. Center for Chemical Process Safety, American Institute of Chemical Engineers, New York.
Alekseev, V.I., Kuznetsov, M.S., Yankin, Y.G. & Dorofeev S.B. (2001) Experimental study of flame acceleration and DDT under conditions of transverse venting. Journal of Loss Prevention in the Processes Industries, 14, pp.591-596.
Aris, R. (1989) Vectors, Tensors, and the Basic Equations of Fluid Mechanics. New York, Dover Publications.
Astbury, G.R. & Hawksworth, S.J. (2007) Spontaneous ignition of hydrogen leaks: a review of postulated mechanisms. International Journal of Hydrogen Energy, 32, pp.2178-2185.
Atkins, P.W. & de Paula, J. (2006) Physical Chemistry. 8th edition. Oxford, Oxford University Press.
Baker, W.E., Cox, P.A., Westine, P.S., Kulesz, J.J., & Strehlow, R.A. (1983) Explosion Hazards and Evaluation, volume 5 of Fundamental studies in engineering. New York, Elsevier.
Barthelemy, H. (2006) Compatibility of metallic materials with hydrogen. A lecture presented at the First European Summer School on Hydrogen Safety, 15-24 August 2006, Belfast, United Kingdom.
Batchelor G.K. (1993) The theory of homogeneous turbulence. Cambridge Science Classics. Cambridge University Press.
Batchelor, G.K. (1994) An introduction to fluid dynamics. Cambridge, Cambridge University Press.
Bird, R.B., Stewart, W.E., & Lightfoot E.N. (2002) Transport phenomena. 2nd edition. New York, Wiley.
Bradley, D. & Mitcheson, A. (1976) Mathematical solutions for explosions in spherical vessels. Combustion and Flame, 26, pp.201-217.
Bradley D. & Mitcheson A. (1978) The venting of gaseous explosions in spherical vessels. I - Theory. Combustion and Flame, 32, pp.221-236.
Bradley, D. & Mitcheson, A. (1978) The venting of gaseous explosions in spherical vessels. II - Theory and experiment. Combustion and Flame, 32, pp.237-255.
Bradley, D. (1992) How fast can we burn? In: Proceedings of the Twenty-Fourth Symposium (International) on Combustion. Pittsburgh, The Combustion Institute. pp.247-262.
Bradley, D., Lawes, M., Scott, M.J., & Mushi, E.M.J. (1994) Afterburning in spherical premixed turbulent explosions. Combustion and Flame, 99, pp.581-590.
Bradley, D. (1999) Instabilities and flame speeds in large-scale premixed gaseous explosions. Philosophical Transactions of the Royal Society of London, Series A, 357, pp.3567-3581.
Bradley, D., Sheppard, C.G.W., Woolley, R., Greenhalgh, D.A., & Lockett, R.D. (2000) The development and structure of flame instabilities and cellularity at low Markstein numbers in explosions. Combustion and Flame, 122, pp.195-209.
Bradley D. Burning rates in gaseous explosions of hydrogen-air. A lecture presented at the First European Summer School on Hydrogen Safety, 15-24 August 2006.
Bradley, D., Lawes, M., Liu, K., Verhelst, S., & Woolley, R. (2007) Laminar burning velocities of lean hydrogen-air mixtures at pressures up to 1.0 MPa. Combustion and Flame, 149, pp.162-172.
Breitung, W., Chan, C.K., Dorofeev, S.B., Eder, A., Gelfand, B.E., Heitsch, M., Klein, R., Malliakos, A., Shepherd, J.E., Studer, E. & Thibault P. (2000) Flame acceleration and deflagration to detonation transition in nuclear safety. State-of-the-art report by a group of experts. August 2000. OECD Nuclear Energy Agency.
Bulent Yuceil, K. & Volkan Otugen, M. (2002) Scaling parameters for underexpanded supersonic jets. Physics of Fluids, 14. pp.4206-4215.
Cant R.S. and Mastorakos E. (2008) An introduction to turbulent reacting flows. London, Imperial College Press.
Casal, J. (2008) Evaluation of the effects and consequences of major accidents in industrial plants. Industrial Safety Series. Amsterdam, Elsevier.
Chelhaoui, S. & Serre Combe, P. (2006) Overview of European and international regulation and standardisation activities. Paper presented at the Sixteenth World Hydrogen Energy Conference,13-16 June 2006, Lyon, France. International Association for Hydrogen Energy.
Chen, C.J. & Rodi W. (1980) Vertical turbulent buoyant jets: a review of experimental data, volume 4 of HMT - Science and Applications of Heat and Mass Transfer. Oxford, Pergamon Press.
Cheng, Z., Agranat, V.M., Tchouvelev, A.V., Houf, W. & Zhubrin, S.V. (2005) PRD hydrogen release and dispersion, a comparison of CFD results obtained from using ideal and real gas law properties. Paper presented at the International Conference on Hydrogen Safety, 8-10 September 2005, Pisa, Italy.
Ciccarelli, G. (2002) Critical tube measurements at elevated initial mixture temperatures. Combustion Science and Technology, 174, pp.173-183.
Committee for the Prevention of Disasters (2005) Methods for determining and processing probabilities, CPR 12E. Publication Series on Dangerous Substances. The Dutch Ministry of the Interior and Kingdom Relations, The Hague, The Netherlands, second edition, 2005. Red Book, 2005 revision of the first edition published in 1997.
Committee for the Prevention of Disasters (1997) Methods for the calculation of physical effects due to releases of hazardous materials (liquids and gases), CPR14E. Publication Series on Dangerous Substances. The Dutch Ministry of the Interior and Kingdom Relations, The Hague, The Netherlands, third edition, 2005. Yellow Book, 2005 revision of the third edition published in 1997.
Committee for the Prevention of Disasters (2005) Methods for the determination of possible damage to people and objects resulting from releases of hazardous materials, CPR 16E. Publication Series on Dangerous Substances. The Dutch Ministry of the Interior and Kingdom Relations, The Hague, The Netherlands, first edition, 1992. Green Book, 2005 revision of the first edition published in 1992.
Committee for the Prevention of Disasters (2005) Guidelines for quantitative risk assessment, CPR 18E. Publication Series on Dangerous Substances. The Dutch Ministry of the Interior and Kingdom Relations, The Hague, The Netherlands, first edition, 2005. Purple Book, 2005 revision of the first edition published in 1999.
Dorofeev, S.B. (1996) Blast effect of confined and unconfined explosions. In: Sturtevant, B., Shepherd, J., and Hornung, H. (eds.) Shock Waves, Proceedings of the 20th ISSW, 1996, volume 1, Singapore. World Scientific Publishing Co. pp.77-86.
Dorofeev, S.B., Sidorov, V.P., & Dvoinishnikov, A.E. (1996) Blast parameters from unconfined gaseous detonations. In Sturtevant, B., Shepherd, J., and Hornung, H. (eds.) Shock Waves, Proceedings of the 20th ISSW, 1996, volume 1, Singapore. World Scientific Publishing Co. pp.673-678.
Dorofeev S. B., Kuznetsov M. S., Alekseev V. I., Efimenko A.A. & Breitung W. (2001) Evaluation of limits for effective flame acceleration in hydrogen mixtures. Journal of Loss Prevention in the Processes Industries, 14, pp.583-589.
Dorofeev, S.B. (2007) Evaluation of safety distances related to unconfined hydrogen explosions. International Journal of Hydrogen Energy, 32, pp.2118-2124.
Drysdale, D. (1999) An Introduction to Fire Dynamics. Chichester, John Wiley & Sons.
European Commission. Council Directive 73/23/EEC of 19 February 1973 on the harmonization of the laws of member states relating to electrical equipment designed for use within certain voltage limits. Official Journal of the European Union, L 77, 26.3.1973:29-38, 1973.
European Commission. Council Directive 89/336/EEC of 3 May 1989 on the approximation of the laws of the Member States concerning electromagnetic compatibility. Official Journal of the European Union, L 139, 23.5.1989:19-26, 1989.
European Commission. Directive 94/9/EC of the European Parliament and of the Council of 23 March 1994 on the approximation of the laws of the Member States concerning equipment and protective systems intended for use in potentially explosive atmospheres. Official Journal of the European Union, L 100, 19.4.1994:1-33, 1994. EU ATEX 100.
European Commission. Directive 98/37/ec of the European Parliament and of the Council of 22 June 1998 on the approximation of the laws of the Member States relating to machinery. Official Journal of the European Union, L 207, 12.08.1998:1-48, 1998.
European Commission. Directive 1999/92/EC of the European Parliament and of the Council of 16 December 1999 on minimum requirements for improving the safety and health protection of workers potentially at risk from explosive atmospheres (15th individual Directive within the meaning of Article 16(1) of Directive 89/391/EEC). Official Journal of the European Union, L 23, 28.1.2000:57-68, 2000. EU ATEX 137.
European Industrial Gases Association. Gaseous hydrogen stations. Technical Report IGC Doc 15/06/E, EIGA, Brussels, 2006. Revision of Doc 15/96 and Doc 15/05.
European Industrial Gases Association. Determination of safety distances. Technical Report IGC Doc 75/01/E/rev, EIGA, Brussels, 2001.
European Industrial Gases Association. Determination of safety distances. Technical Report IGC Doc 75/01/E/rev, EIGA, Brussels, 2007. Revision of Doc 75/01/rev.
European Industrial Gases Association. Hydrogen cylinders and transport vessels. Technical Report IGC Doc IGC Doc 100/03/E, EIGA, Brussels, 2003. Revision of TN 26/81.
Fickett, K.K. & Davis, W.C. (2001) Detonation: theory and experiment. New York, Dover.
Griffiths, J.F. & Barnard, J.A. (1995) Flame and Combustion. 3rd edition. London, Chapman & Hall.
Grossel, S.S. (2007) Deflagration and detonation flame arresters. New York, Center for Chemical Process Safety of the American Institute of Chemical Engineers.
Hinze J.O. (1975) Turbulence. McGraw-Hill Series in Mechanical Engineering, New York, second edition.
Hirschfelder J.O., Curtiss C.F., and Bird R.B. (1967) The molecular theory of gases and liquids. Fourth Edition. John Wiley & Sons, New York.
Houf, W. & Schefer, R. (2007) Predicting radiative heat fluxes and flammability envelopes from unintended releases of hydrogen. International Journal of Hydrogen Energy, 32, pp.136-141.
Hunt G.R. and Linden P.F. (2001) Steady-state flows in an enclosure ventilated buoyancy forces assisted by wind. Journal of Fluid Mechanics, 426:355-386.
Hunt G.R. and Kaye N.G. (2001) Virtual origin correction for lazy turbulent plumes. Journal of Fluid Mechanics, 435:377-396.
HyApproval WP2 (2007). Handbook for hydrogen refuelling station approval. Technical Report Deliverable 2.2, Version 2.0, HyApproval Consortium, www.hyapproval.org, December 2007. Prepared under under FP6 Priority 1.6, Contract Number SES6 - 019813.
Incropera, F.P., De Witt, D.P., Bergman, T.L. & Lavine, A.S. (2006) Fundamentals of Heat and Mass Transfer. 6th edition. New York, John Wiley & Sons.
International Standardization Organization (ISO), ISO TR 15916(E). Basic considerations for the safety of hydrogen systems. First Edition. Reference number ISO TR 15916:2004(E). The International Organization for Standardization, 2004. International Standard, Prepared by Technical Committee ISO/TC 197 Hydrogen Technologies.
International Standardization Organization (ISO), ISO DIS16110-1. Hydrogen generators using fuel processing technologies - Part 1: Safety. The International Organization for Standardization, 2007. International Standard, Prepared by Technical Committee ISO/TC 197 Hydrogen Technologies.
International Standardization Organization (ISO), ISO DIS/CD 16111. Transportable gas storage devices - Hydrogen absorbed in reversible metal hydride. The International Organization for Standardization, 2005. Draft International Standard, Prepared by Technical Committee ISO/TC 197 Hydrogen Technologies.
International Standardization Organization (ISO), ISO DIS22734-1. Hydrogen generators using water electrolysis process - Part 1: Industrial and commercial applications. The International Organization for Standardization, 2005. Draft International Standard, Prepared by Technical Committee ISO/TC 197 Hydrogen Technologies.
International Standardization Organization (ISO), ISO FDIS17268:2006(E). Compressed hydrogen surface vehicle refuelling connection devices. The International Organization for Standardization, 2006. Draft International Standard, Prepared by Technical Committee ISO/TC 197 Hydrogen Technologies.
International Standardization Organization (ISO), ISO PDTS 20012. Gaseous hydrogen - Fuelling stations. The International Organization for Standardization, 2007. Draft International Standard, Prepared by Technical Committee ISO/TC 197 Hydrogen Technologies.
Jordan, T. (2006) Hydrogen as an energy carrier. A lecture presented at the First European Summer School on Hydrogen Safety, 15-24 August 2006, Belfast, United Kingdom.
Kreith, F. & Bohn, M.S. (2001) Principles of heat transfer. 6th edition. Pacific Grove, California, Brooks/Cole Publishers.
Kuhl A.L., Kamel M.M., and Oppenheim A.K. (1972) Pressure waves generated by steady flames. In Proceedings of the Fourteenth Symposium (International) on Combustion, pages 1201-1215, Pittsburgh. The Combustion Institute.
Kundu, K.P. & Cohen, I.M. (2004) Fluid Mechanics. 3rd edition. Amsterdam, Elsevier Academic Press.
Kuo, K.K. (2005) Principles of Combustion. 2nd edition. New York, John Wiley & Sons.
Kuo, K.K. and Acharya R. (2012) Fundamentals of Turbulent and Multi-Phase Combustion. New York, John Wiley & Sons.
Kuo, K.K. and Acharya R. (2012) Applications of Turbulent and Multi-Phase Combustion. New York, John Wiley & Sons.
Lewis, B. & von Elbe, G. (1987) Combustion, Flames and Explosions of Gases. 3rd edition. Academic Press.
Law, C.K. (2006) Combustion Physics. New York, Cambridge University Press.
Law, C.K. (2006) Propagation, structure, and limit phenomena of laminar flames at elevated pressures. Combustion Science and Technology, 178, pp.335-360.
Lee, J.H.S. & Berman, M. (1997) Hydrogen combustion and its application to nuclear reactor safety. In: Greene, G.A., Hartnett, J.P., Irvine Jr., T.F. & Cho, Y.I. (eds.), Heat Transfer in Nuclear Reactor Safety, volume 29 of Advances in Heat Transfer, chapter 2. New York, Academic Press. pp.59-123.
Lee, J.H.S. (2008) The Detonation Phenomenon. New York, Cambridge University Press.
Lees F.P. (1996) Loss Prevention in the Process Industry, volumes 1, 2 & 3. 2nd edition. London, Butterworth.
Lewis, B. & von Elbe, G. (1987) Combustion, Flames and Explosions of Gases. 3rd edition. Academic Press.
Lighthill, M.J. (1978) Waves in fluids. Cambridge, Cambridge University Press.
Milne-Thomson L.M. (1966) Theoretical Aerodynamics. Dover Publications, Inc., New York, fourth edition.
Milne-Thomson L.M. (1968) Theoretical Hydrodynamics. MacMillan Press, New York, fifth edition.
Molkov, V.V., Makarov, D. & Grigorash A. (2004) Cellular structure of explosion flames: modelling and large-eddy simulation. Combustion Science and Technology, 176, pp.851-865.
Molkov, V., Makarov, D. & Schneider, H. (2006) LES modelling of an unconfined large-scale hydrogen-air deflagration. Journal of Physics D: Applied Physics, 39, pp.4366-4376.
NASA. Safety Standard for Hydrogen and Hydrogen Systems (1997). Guidelines for hydrogen system design, materials selection, operations, storage, and transportation. Technical Report NSS 1740.16. Washington, Office of Safety and Mission Assurance.
Nettleton M.A. (1987) Gaseous Detonations: their nature, effects and control. Chapman and Hall. Newsholme G. (2007) The management of risk. A lecture contributed to Module Principles of Hydrogen Safety of the Postgraduate Certificate in Hydrogen Safety Engineering. Bootle, United Kingdom, The Health and Safety Executive.
Newsholme, G (2007). Hydrogen Safety and Regulation. A lecture contributed to Module Applied Hydrogen Safety of the Postgraduate Certificate in Hydrogen Safety Engineering. Bootle, United Kingdom, The Health and Safety Executive.
NFPA 50A (1999). Standard for gaseous hydrogen systems at consumer sites. 1999 edition. Quincy, MA, United States of America, National Fire Protection Association.
NFPA 55 (1998). Standard for the storage, use, and handling of compressed and liquefied gases in portable cylinders. 1998 edition. Quincy, MA, United States of America, National Fire Protection Association.
NFPA 55 (2005) Standard for the storage, use, and handling of compressed and liquefied gases in portable cylinders. 2005 edition. Quincy, MA, United States of America, National Fire Protection Association.
NFPA 853 (2003) Standard for the installation of stationary fuel cell power plants, 2003 edition. Quincy, MA, United States of America, National Fire Protection Association.
Ng H.D., Ju Y., and Lee J.H.S. Assessment of detonation hazards in high-pressure hydrogen storage from chemical sensitivity analysis. International Journal of Hydrogen Energy, 32:93-99, 2007.
Ngo, T., Mendis, P., Gupta, A. & Ramsay, J. (2007) Blast loading and blast effects on structures - An overview. Electronic Journal of Structural Engineering, 7, pp.76-91. Special Issue: Loading on Structures.
Oppenheim A.K. (2006) Dynamics of Combustion Systems. Springer-Verlag, New York.
Pasman, H.J. (2006) The challenge of risk control in a hydrogen based economy. A lecture presented at the First European Summer School on Hydrogen Safety, 15-24 August 2006, Belfast, United Kingdom.
Poinsot, T. & Veynante, D. (2005) Theoretical and numerical combustion. 2nd edition. Philadelphia, Edwards.
Pope, S.B. (2000) Turbulent flows. Cambridge, United Kingdom, Cambridge University Press.
Quintiere, J.G. (2006) Fundamentals Of Fire Phenomena. Chichester, John Wiley & Sons.
Sagaut P. and Germano M. (2002) Large Eddy Simulation for Incompressible Flows: An Introduction. Second Edition. Springer, Berlin.
Schefer, R.W., Houf, W.G., San Marchi, C., Chernicoff, W.P. & Englom, L. (2006) Characterization of leaks from compressed hydrogen dispensing systems and related components. International Journal of Hydrogen Energy, 31, pp.1247-1260.
Schlichting, H. (1968) Boundary-layer theory. 6th edition. Trans. Kestin J. McGraw-Hill Series in Mechanical Engineering. New York, McGraw-Hill.
Shepherd J.E., Moen I.O., Murray S.B., and Thibault P.A. (1986) Analyses of the cellular structure of detonations. In Proceedings of the Twenty-First Symposium (International) on Combustion, pages 1649-1658, Pittsburgh. The Combustion Institute.
Shepherd, J.E. (2006) Elastic and plastic structural response of tubes to deflagration-to-detonation transition. Technical Report Explosion Dynamics Laboratory Report FM2006-00x, Graduate Aeronautical Laboratries, California Institute of Technology, Pasadena, CA 91125, July 2006. Presented at the First European Summer School on Hydrogen Safety, 15-24 August 2006, Belfast, United Kingdom.
Shepherd, J.E. (2006) Structural response of piping to internal gas detonation. In: Proceedings of the ASME Pressure Vessels and Piping Division Conference, July 23-27, 2006, Vancouver BC, Canada. PVP2006-ICPVT-11-93670.
Smith, J.M., Van Ness, H.C. & Abbott, M.M. (2001) Introduction to Chemical Engineering Thermodynamics. 6th edition. New York: McGraw-Hill.
Sonntag, R.E., Borgnakke, C. & van Wylen, G.J. (2003) Fundamentals of Thermodynamics. 6th edition. New York, John Wiley & Sons 2003.
Stamps, D.W., Slezak, S.E., & Tiezen, S.R. (2006) Observations of the cellular structure of fuel-air detonations. Combustion and Flame, 144, pp.289-298.
Swain, M.R. & Swain, M.N. (1996) Passive ventilation systems for the safe use of hydrogen. International Journal of Hydrogen Energy, 21, pp823-835.
Swain, M.R., Filoso, P., Grilliot, E.S. & Swain, M.N. (2003) Hydrogen leakage into simple geometric enclosures. International Journal of Hydrogen Energy, 28, pp.229-248.
Tang, M.J. & Baker, Q.A. (1999) A new set of blast curves from vapor cloud explosion. Process Safety Progress, 18, pp.235-240.
Tang, M.J. & Baker, Q.A. (2000) Comparison of blast curves from vapor cloud explosions. Journal of Loss Prevention in the Processes Industries, 13, pp.433-438.
Teodorczyk, A. (2006) Fast deflagrations, deflagration to detonation transition (DDT) and direct detonation in hydrogen-air mixtures. A lecture presented at the First European Summer School on Hydrogen Safety, 15-24 August 2006, Belfast, United Kingdom.
Thomas, G.O. (2002) The response of pipes and supports to internal pressure loads generated by gaseous detonations. Journal of Pressure Vessel Technology, Transactions of the ASME, 124, pp.66-73.
Tieszen S.R., Stamps D.W., Westbrook C.K., and Pitz W.J. (1991) Gaseous hydrocarbon-air detonations. Combustion and Flame, 84:376-390.
Tse, S.D., Zhu, D.L. & Law, C.K. (2000) Morphology and burning rates of expanding spherical flames in H2/O2/inert mixtures up to 60 atmospheres. In: Proceedings of the Twenty-Eighth Symposium (International) on Combustion. Pittsburgh, The Combustion Institute. pp.1793-1800.
Verfondern, K. & Dienhart B. (1997) Experimental and theoretical investigation of hydrogen pool spreading and vaporization. International Journal of Hydrogen Energy, 22, pp.649-660.
Verfondern, K. & Dienhart, B. (2007) Pool spreading and vaporization of liquid hydrogen. International Journal of Hydrogen Energy, 32, pp.256-267.
Venetsanos, A.G., Huld, T., Adams, P., & Bartzis, J.G. (2003) Source, dispersion and combustion modelling of an accidental release of hydrogen in an urban environment. Journal of Hazardous Materials, A105, pp.1-25.
Venetsanos, A.G. & Bartzis, J.G. (2007) CFD modeling of large-scale LH2 spills in open environment. International Journal of Hydrogen Energy, 32, pp.2171-2177.
Venetsanos, A.G., Baraldi, D., Adams, P., Heggem, P.S. & Wilkening, H. (2008) CFD modelling of hydrogen release, dispersion and combustion for automotive scenarios. Journal of Loss Prevention in the Processes Industries, 21, pp.162-184.
Warnatz, J., Maas, U., & Dibble, R.W. (2005) Combustion: Physical and Chemical Fundamentals, Modeling and Simulation, Experiments, Pollutant Formation. 3rd edition. New York, Springer.
Wen, J.X. (2006) Hydrogen fires. A lecture presented at the First European Summer School on Hydrogen Safety, 15-24 August 2006, Belfast, United Kingdom.
Westbrook C.K. and Urtiew P.A. (1982) Chemical kinetic prediction of critical parameters in gaseous detonation. In Proceedings of the Nineteenth Symposium (International) on Combustion, pages 615-623, Pittsburgh. The Combustion Institute.
Williams, F.A. (1985) Combustion Theory: the fundamental theory of chemically reacting flow systems. 2nd edition. Combustion Science and Engineering Series. Menlo Park, California, The Benjamin/Cummings Publishing Company.
Williams, F.A. (2006) Reduced chemistry for hydrogen combustion and detonation. A lecture presented at the First European Summer School on Hydrogen Safety, 15-24 August 2006, Belfast, United Kingdom.
Williams, F.A. (2008) New developments in the understanding of hydrogen laminar burning velocities and spontaneous ignition. A lecture presented at the Third European Summer School on Hydrogen Safety, 21-31 July 2008, Belfast, United Kingdom.
Wurster, R. (2006) HyApproval - Handbook for approval of hydrogen refuelling stations - Safe and harmonized implementation of hydrogen refuelling stations on a global scale. A lecture presented at the First European Summer School on Hydrogen Safety, 15-24 August 2006, Belfast, United Kingdom.
Zalosh, R. (2006) Hydrogen mixing in large enclosures. A lecture presented at the First European Summer School on Hydrogen Safety, 15-24 August 2006, Belfast, United Kingdom.